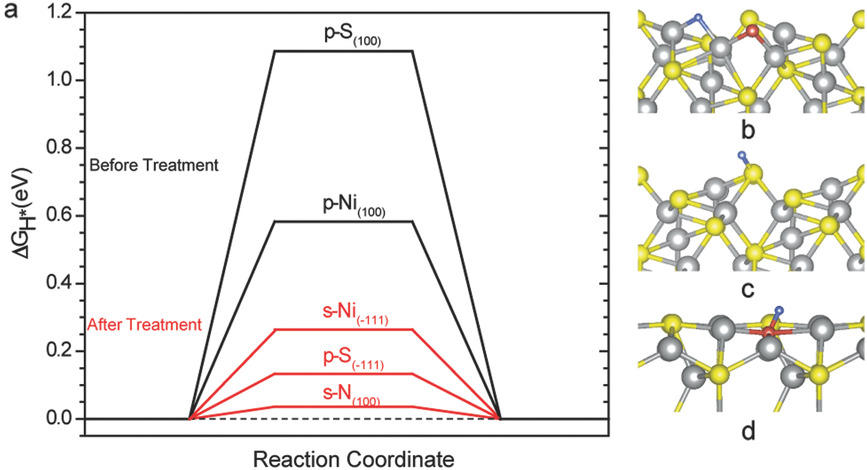
Nickel sulfide (Ni3S2) is a promising hydrogen evolution reaction (HER) catalyst by virtue of its metallic electrical conductivity and excellent stability in alkaline medium. However, the reported catalytic activities for Ni3S2 are still relatively low. Herein, an effective strategy to boost the H adsorption capability and HER performance of Ni3S2 through nitrogen (N) doping is demonstrated. N‐doped Ni3S2 nanosheets achieve a fairly low overpotential of 155 mV at 10 mA cm−2 and an excellent exchange current density of 0.42 mA cm−2 in 1.0 m KOH electrolyte. The mass activity of 16.9 mA mg−1 and turnover frequency of 2.4 s−1 obtained at 155 mV are significantly higher than the values reported for other Ni3S2‐based HER catalysts, and comparable to the performance of best HER catalysts in alkaline medium. These experimental data together with theoretical analysis suggest that the outstanding catalytic activity of N‐doped Ni3S2 is due to the enriched active sites with favorable H adsorption free energy. The activity in the Ni3S2 is highly correlated with the coordination number of the surface S atoms and the charge depletion of neighbor Ni atoms. These new findings provide important guidance for future experimental design and synthesis of optimal HER catalysts.
Tianyi Kou, Tyler Smart, Bin Yao, Irwin Chen, David Thota, Yuan Ping, and Yat Li, “Theoretical and Experimental Insight into the Effect of Nitrogen Doping on Hydrogen Evolution Activity of Ni3S2 in Alkaline Medium”, Adv. Energy Mater, 1703538 (2018). Link to the article